STRUCTURAL MANIFESTATION OF THE FUNCTIONAL HIPPOCAMPO-CORTICAL CONNECTIONS IN HUMANS
1Department of Medicine, Harvard University, Cambridge, United States
*Corresponding Author:
Mustapha Akhdar, Department of Medicine, Harvard University, Cambridge,
United States,
Email: aseanjournalofpsychiatry.ajopy@gmail.com
Received: 31-Jul-2023, Manuscript No. AJOPY-23-108627;
Editor assigned: 03-Aug-2023, Pre QC No. AJOPY-23-108627 (PQ);
Reviewed: 17-Aug-2023, QC No. AJOPY-23-108627;
Revised: 15-Jan-2025, Manuscript No. AJOPY-23-108627 (R);
Published:
22-Jan-2025, DOI: 10.54615/2231-7805.47390
Abstract
The human brain is a complex network of interconnected regions that collaborate to support cognitive processes and memory formation. The hippocampus, a crucial hub for episodic memory and spatial navigation, and the cortical areas, responsible for higher-order cognitive functions, are intricately intertwined. These hippocampal-cortical connections suggest that asymmetry in the hippocampal neuronal activity may directly influence the formation of synaptic connections among cortical neurons, thus modulating the processes of neuroplasticity and triggering the formation of cortical functional and morphological asymmetry. This research paper aims to provide a comprehensive overview of the association between hippocampus size and cortical area sizes, shedding light on their synergistic interplay in supporting cognitive processes. Four human brain specimens (2 male, 2 female) were utilized, and the dimensions of the hippocampus and specific cortical gyri were measured using the Einscan H 3D laser scanner. The results revealed asymmetry and variability in sizes across brain regions and hemispheres. Specifically, the right hemisphere exhibited larger hippocampal size compared to the left hemisphere, with the frontal gyri predominantly larger on the right side. Additionally, the right hippocampus showed significant correlations with contralateral inferior and middle frontal gyri, while the left hippocampus displayed pronounced correlations with contralateral middle and superior lateral frontal gyri. The findings highlight the influential role of the hippocampus in shaping cortical area sizes, particularly in the frontal gyri. These results contribute to our understanding of the complex interdependence between the hippocampus and cortical areas, with implications for memory-related disorders and cognitive deficits. Further research is needed to explore the developmental and aging changes in hippocampal-cortical connections and their impact on brain function.
Keywords
Hippocampus, Cortical areas, 3D scanning, Size, Memory, Cognition, Interdependence
Introduction
The human brain, a marvel of complexity, comprises
various interconnected regions that collaborate to support
cognitive processes and facilitate memory formation and
retrieval. Among these regions, the hippocampus and
cortical areas emerge as vital players, intricately
intertwined in a dynamic network. The hippocampus,
located in the medial temporal lobe, has long been
recognized as a critical center for episodic memory and
spatial navigation. Meanwhile, cortical areas, distributed
across the cerebral cortex, are responsible for higherorder
cognitive functions, including perception,
language, attention, and executive control [1].
Understanding the nature of the relationship between the
hippocampus and cortical areas is pivotal to unraveling
the fundamental mechanisms underlying memory and
cognition. This research paper aims to provide a
comprehensive overview of the association between
hippocampus size with cortical area sizes. It delves into
the complex connections between those two structures
shedding light on their synergistic interplay in supporting
cognitive processes and influencing the measurements of
these two vital areas [2]. By synthesizing findings from
the Einscan 3D scanner this paper aims to emphasize the
role of the hippocampus in influencing the size of
different cortical areas. Through an integrative approach,
this research endeavors to enhance our comprehension of
the complex interdependence between the hippocampus
and cortical areas, providing valuable insights into the
broader framework of cognitive neuroscience.
Ultimately, this exploration may pave the way for novel
avenues of research and clinical interventions targeting
memory-related disorders and cognitive deficits [3]. The objective of this research is to uncover the structural
manifestations of the functional connections between the
hippocampus and cortical areas [4]. It aims to establish
morphological correlations between the volume of
specific brain regions (frontal, temporal, parietal, and
occipital gyri) and the dimensions of the hippocampus on
both the same and opposite side of the brain [5].
Materials and Methods
Four specimens of the human brain (2-male; 2-female) without visually detected malformation and pathology were included in our study. Brains 1 and 2 were the male brains while brains 3 and 4 were the female brains. General dissection tools were applied for anatomical dissection; an electric saw was used for craniotomies; EinScan H 3D laser scanner was used for the building of 3D models; 3D Shining software was used for the measuring on the 3D models; IBM SPSS software was applied for the statistical analysis of the obtained data [6]. The data was obtained on 4 brain models involving left and right hemispheres. The research was approved by the ethical committee [7].
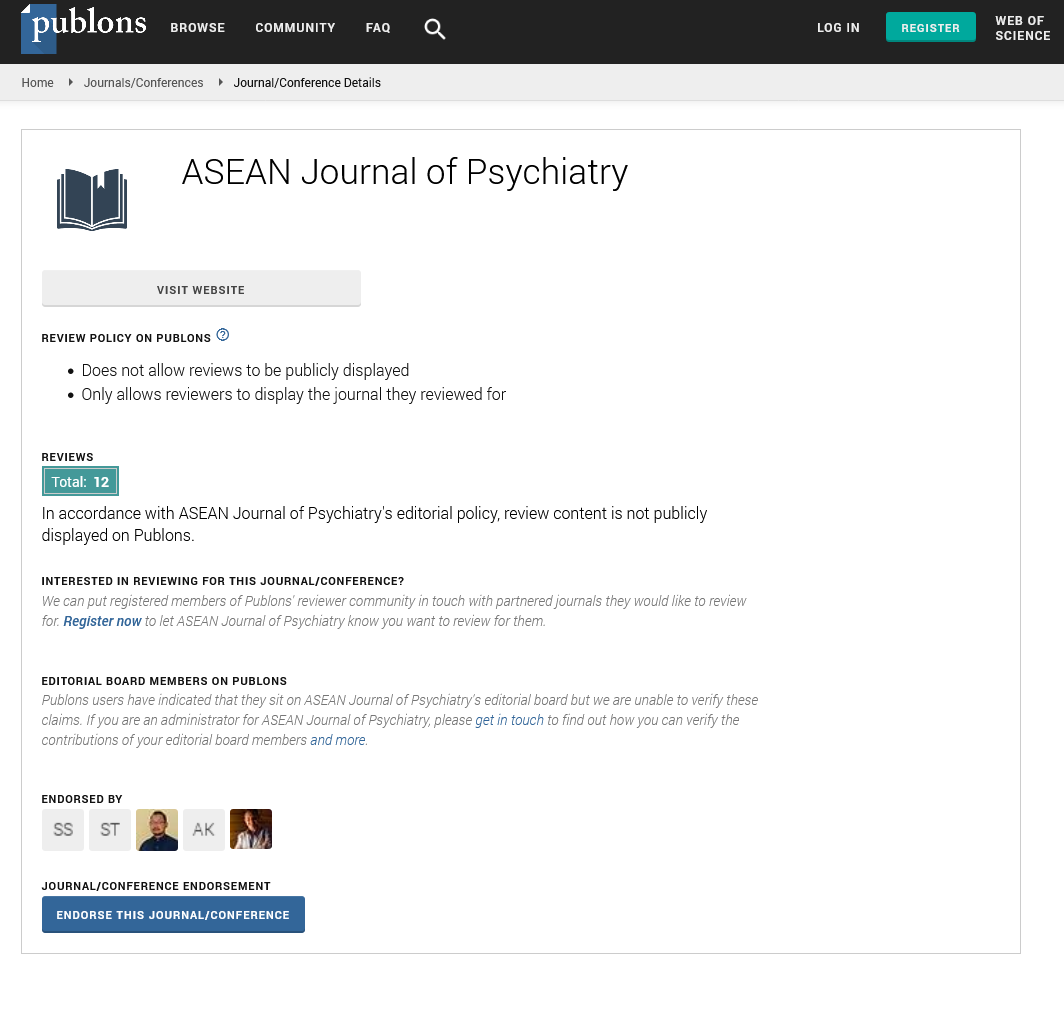
• After the routine educational craniotomy, the brain was extracted from the cranial cavity and its surface was cleaned from the arachnoid mater and cerebral vessels while closely avoiding any damage to the gyri. The brainstem was dissected at the level of the midbrain to expose the parahippocampal gyrus and uncus of the ventral surface of the cerebral hemispheres (Figure 1).
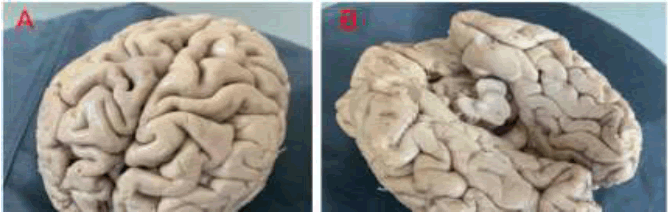
• Then, the cerebral hemispheres are positioned on the rotatory table of the EinScan 3D scanner. The blue light 3D scanner was used to digitize the cerebral hemispheres and the “Shining 3D” application was employed to measure the surface areas of all gyri [8]. The difference in the light angles and reflection points on photos are analyzed via the software to build the 3D model of the specimen. Then the temporal lobes of the brain are dissected. The floor of the lateral ventricles with the hippocampus and dentate gyrus in the bottom is exposed and cleaned from the choroid plexuses (Figure 2). The scaling and rotation settings allow to set up the accurate dimensions of the structures with a maximum of 0.07 px residual level [9].
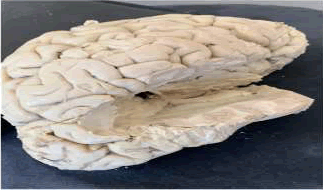
•The 3D scanner obtained two scans of the hemispheres one from the surface of the lateral ventricle towards the surface of the cerebral hemisphere and another from the bottom of the lateral ventricle towards the top of the lateral ventricle (Figure 3). Then we began measuring the different cortical areas using the Shining 3D software by slowly highlighting the area and allowing the software to calculate the surface area [10].
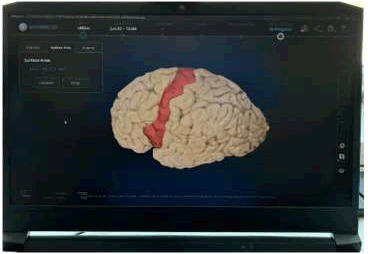
• Following the digitization of the surface areas, the cortex of the temporal and occipital lobes was dissected revealing the hippocampus at the floor of the lateral ventricle, and the surface area of the hippocampus, and parahippocampal formation were measured as well [11]. After measuring all the different cortical areas we went on to measure the hippocampus and used the distance tool instead of the surface area tool [12]. We measured the head, body and tail of the hippocampus [13]. After obtaining those measurements we measured the surface area for the hippocampus (Figure 4) [14].
The descriptive statistics and correlation analysis were performed using the IBM SPSS software to identify the general morphological tendencies and statistically significant relationships between the morphological parameters of the hippocampus and the ipsi- and contralateral cortical gyri in males and females [15].
Standard protocol approvals, registrations, and patient consents
There was no potential harm to participants. Work was performed following safety rules and regulations set by the anatomy and neurology department leadership [16].
Data availability
Anonymized data not published within this article will be made available by request from any qualified investigator [17].
Results
In general, the right hemisphere of the brain exhibited larger hippocampal size compared to the left hemisphere. The measurements of cortical areas on the right hemisphere were mostly greater than those on the left hemisphere. Notably, the occipital gyri were predominantly larger in the left hemisphere, except for brain 4 where the measurements were very close on both sides. On the other hand, the frontal gyri tended to be larger in the right hemisphere. The right hippocampi were generally larger than the left hippocampi, except in the case of brain 3 where the left hippocampus was larger. Furthermore, brains 1 and 4 exhibited predominantly larger left temporal gyri. These findings highlight the asymmetry and variability in the sizes of hippocampus and cortical areas across different hemispheres and brain regions where the right hemispheres have larger frontal gyri while the left hemispheres have larger temporal gyri (Tables 1-8).
| Brain 1-Left |
Brain 1-Right |
| |
|
Surface area mm2 |
|
|
Surface area mm2 |
| Frontal gyri |
Precentral |
1,991.33 |
Frontal gyri |
Precentral |
2185.55 |
| Superior lateral frontal |
3536.85 |
Superior lateral frontal |
2644.47 |
| Superior medial frontal |
2440.37 |
Superior medial Frontal |
2461.23 |
| Middle frontal |
1952.92 |
Middle fronsstal |
2325.01 |
| Inferior frontal |
1328.28 |
Inferior frontal |
1663.87 |
| Straight gyrus |
493.13 |
Straight gyrus |
489.31 |
| Anterior orbital |
444.06 |
Anterior orbital |
495.79 |
| Posterior orbital |
622.44 |
Posterior orbital |
572.99 |
| Lateral orbital |
386.38 |
Lateral orbital |
443.79 |
| Medial orbital |
569.78 |
Medial orbital |
533.11 |
| Temporal gyri |
Superior temporal |
1345 |
Temporal gyri |
Superior temporal |
1118.57 |
| Middle temporal |
2410.99 |
Middle temporal |
2018.93 |
| Inferior temporal |
2116.85 |
Inferior temporal |
2315.88 |
| Fusiform gyrus |
2776.68 |
Fusiform gyrus |
2582.21 |
| Parahippocampa |
929.48 |
Parahippocampal |
899.33 |
| Uncus |
146.26 |
Uncus |
122.02 |
| Parietal lobe |
Postcentral |
2361.97 |
Parietal lobe |
Postcentral |
2339.21 |
| Superior parietal |
3678.74 |
Superior parietal |
3098.74 |
| Inferior parietal |
2058.25 |
Inferior parietal |
2112.7 |
| Precuneus |
1641.55 |
Precuneus |
1643.24 |
| Occipital lobe |
Occipital gyrus |
2014.45 |
Occipital lobe |
Occipital gyrus |
1972.19 |
| Lingual gyrus |
778.56 |
Lingual gyrus |
742.66 |
| Cuneus |
1774.64 |
Cuneus |
1739.87 |
| Hippocampus |
Head |
19.057 mm |
Hippocampus |
Head |
16.94 mm |
| Body |
15.25 mm |
Body |
13.23 mm |
| Tail |
16.075 mm |
Tail |
11.32 mm |
| Area |
858.64 mm2 |
Area |
1060.51 mm2 |
Table 1. Measurements of brain 1 cortical areas and hippocampus. (left and right hemispheres).
| Brain 2-Left |
Brain 2-Right |
| |
|
Surface area mm2 |
|
|
Surface area mm2 |
| Frontal gyri |
Precentral |
1676.43 |
Frontal gyri |
Precentral |
1868.8 |
| Superior lateral frontal |
2806.91 |
Superior lateral frontal |
2044.5 |
| Superior medial Frontal |
2547.66 |
Superior medial Frontal |
2808.84 |
| Middle frontal |
1849.12 |
Middle frontal |
1970.51 |
| Inferior frontal |
1205.64 |
Inferior frontal |
1351.35 |
| Straight gyrus |
578.39 |
Straight gyrus |
542.79 |
| Anterior orbital |
495.15 |
Anterior orbital |
330.5 |
| Posterior orbital |
453.6 |
Posterior orbital |
437.37 |
| Lateral orbital |
403.14 |
Lateral orbital |
594.58 |
| Medial orbital |
528.79 |
Medial orbital |
552.01 |
| Temporal gyri |
Superior temporal |
1552.94 |
Temporal gyri |
Superior temporal |
1621.9 |
| Middle temporal |
2263.01 |
Middle temporal |
2176.51 |
| Inferior temporal |
1822.82 |
Inferior temporal |
2252.55 |
| Fusiform gyrus |
2251.15 |
Fusiform gyrus |
2408.75 |
| Parahippocampal |
899.56 |
Parahippocampal |
731.34 |
| Uncus |
197.98 |
Uncus |
234.45 |
| Parietal lobe |
Postcentral |
1774.87 |
Parietal lobe |
Postcentral |
2056.65 |
| Superior parietal |
3038 |
Superior parietal |
2971.75 |
| Inferior parietal |
2357.28 |
Inferior parietal |
2025.86 |
| Precuneus |
1703.61 |
Precuneus |
1631.57 |
| Occipital lobe |
Occipital gyrus |
1855.28 |
Occipital lobe |
Occipital gyrus |
1874.32 |
| Lingual gyrus |
855.82 |
Lingual gyrus |
741.17 |
| Cuneus |
1727.43 |
Cuneus |
1618.13 |
| Hippocampus |
Head |
16.94 mn |
Hippocampus |
Head |
18.14 mm |
| Body |
15.3 mm |
Body |
13.94 mm |
| Tail |
17.37 mm |
Tail |
15.24 mm |
| Area |
952.97 mm2 |
Area |
1038.96 mm2 |
Table 2. Measurements of brain 2 cortical areas and hippocampus. (left and right hemispheres).
| Brain 3-Left |
Brain 3-Right |
| |
|
Surface area mm2 |
|
|
Surface area mm2 |
| Frontal gyri |
Precentral |
1913.55 |
Frontal gyri |
Precentral |
2093.48 |
| Superior lateral frontal |
3098.47 |
Superior lateral frontal |
3108.65 |
| Superior medial frontal |
2418.95 |
Superior medial Frontal |
23337.97 |
| Middle frontal |
2085.88 |
Middle frontal |
2145.93 |
| Inferior frontal |
1566.11 |
Inferior frontal |
1655.58 |
| Straight gyrus |
506.63 |
Straight gyrus |
459,71 |
| Anterior orbital |
388.09 |
Anterior orbital |
422.12 |
| Posterior orbital |
565.01 |
Posterior orbital |
448,9 |
| Lateral orbital |
429.99 |
Lateral orbital |
476.44 |
| Medial orbital |
541.04 |
Medial orbital |
574.72 |
| Temporal gyri |
Superior temporal |
1250.06 |
Temporal gyri |
Superior temporal |
1563.36 |
| Middle temporal |
2105.26 |
Middle temporal |
2125.12 |
| Inferior temporal |
2024.98 |
Inferior temporal |
1988.06 |
| Fusiform gyrus |
2370.87 |
Fusiform gyrus |
2178.65 |
| Parahippocampal |
883.33 |
Parahippocampal |
808.42 |
| Uncus |
175.19 |
Uncus |
197.72 |
| Parietal lobe |
Postcentral |
2082.8 |
Parietal lobe |
Postcentral |
1965.96 |
| Superior parietal |
3119.96 |
Superior parietal |
2914.81 |
| Inferior parietal |
2261.16 |
Inferior parietal |
2116.81 |
| Precuneus |
1534.9 |
Precuneus |
1575.31 |
| Occipital lobe |
Occipital gyrus |
1852.19 |
Occipital lobe |
Occipital gyrus |
1966.45 |
| Lingual gyrus |
795,54 |
Lingual gyrus |
737.46 |
| Cuneus |
1536.44 |
Cuneus |
1430.14 |
| Hippocampus |
Head |
14.28 mm |
Hippocampus |
Head |
13.41 mm |
| Body |
14.94 mm |
Body |
13.78 mm |
| Tail |
17.32 mm |
Tail |
16.36 mm |
| Area |
871.22 mm2 |
Area |
741.16 mm2 |
Table 3. Measurements of brain 3 cortical areas and hippocampus. (left and right hemispheres).
| Brain 4-Left |
Brain 4-Right |
| |
|
Surface area mm2 |
|
|
Surface area mm2 |
| Frontal gyri |
Precentral |
1992.04 |
Frontal gyri |
Precentral |
1863.62 |
| Superior lateral frontal |
3025.91 |
Superior lateral frontal |
3178.68 |
| Superior medial frontal |
2332.8 |
Superior medial frontal |
2120.51 |
| Middle frontal |
2074.88 |
Middle frontal |
2261.45 |
| Inferior frontal |
1528.57 |
Inferior frontal |
1646.25 |
| Straight gyrus |
470.91 |
Straight gyrus |
370.09 |
| Anterior orbital |
420.94 |
Anterior orbital |
403.81 |
| Posterior orbital |
495.69 |
Posterior orbital |
527.34 |
| Lateral orbital |
441.89 |
Lateral orbital |
418.71 |
| Medial orbital |
561.47 |
Medial orbital |
549.09 |
| Temporal gyri |
Superior temporal |
1330.87 |
Temporal gyri |
Superior temporal |
1451.63 |
| Middle temporal |
2067.8 |
Middle temporal |
1979.15 |
| Inferior temporal |
2160.06 |
Inferior temporal |
1946.76 |
| Fusiform gyrus |
2158.9 |
Fusiform gyrus |
2179.39 |
| Parahippocampal |
826.01 |
Parahippocampal |
724,51 |
| Uncus |
175.98 |
Uncus |
159.88 |
| Parietal lobe |
Postcentral |
1942.31 |
Parietal lobe |
Postcentral |
1978.18 |
| Superior parietal |
3014.51 |
Superior parietal |
3129.46 |
| Inferior parietal |
2222.8 |
Inferior parietal |
2227,53 |
| Precuneus |
1507.34 |
Precuneus |
1574.71 |
| Occipital lobe |
Occipital gyrus |
1833.36 |
Occipital lobe |
Occipital gyrus |
1870.43 |
| Lingual gyrus |
723.06 |
Lingual gyrus |
677.82 |
| Cuneus |
1304.97 |
Cuneus |
1342.29 |
| Hippocampus |
Head |
17 |
Hippocampus |
Head |
18 |
| Body |
17 |
Body |
13 |
| Tail |
16 |
Tail |
16 |
| Area |
675 |
Area |
840 |
Table 4. Measurements of brain 4 cortical areas and hippocampus. (left and right hemispheres).
| |
|
Hippocampus surface area L mm2 |
Straight gyrus R |
Straight gyrus L |
Anterior orbital R |
Anterior orbital L |
Posterior orbital R |
Posterior orbital L |
Lateral orbital R |
Lateral orbital L |
Medial orbital R |
| Hippocampus Surface area L mm2 |
Pearson correlation |
1 |
0.972 |
0.841 |
-0.25 |
0.494 |
-0.519 |
-0.006 |
0.813 |
-0.658 |
0.139 |
| Sig. (2-tailed) |
|
0.028 |
0.159 |
0.75 |
0.506 |
0.481 |
0.994 |
0.187 |
0.342 |
0.861 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Straight gyrus R |
Pearson correlation |
.972 |
1 |
0.857 |
-0.243 |
0.663 |
-0.386 |
-0.039 |
0.821 |
-0.777 |
-0.081 |
| Sig. (2-tailed) |
0.028 |
|
0.143 |
0.757 |
0.337 |
0.614 |
0.961 |
0.179 |
0.223 |
0.919 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Straight gyrus L |
Pearson correlation |
0.841 |
0.857 |
1 |
-0.709 |
0.741 |
-0.707 |
-0.538 |
.998" |
-0.408 |
0.148 |
| Sig. (2-tailed) |
0.159 |
0.143 |
|
0.291 |
0.259 |
0.293 |
0.462 |
0.002 |
0.592 |
0.852 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Anterior orbital R |
Pearson correlation |
-0.25 |
-0.243 |
-0.709 |
1 |
-492 |
0.796 |
0.96 |
-0.752 |
-0.292 |
-0.381 |
| Sig. (2-tailed) |
0.75 |
0.757 |
291 |
|
0.508 |
0.204 |
0.04 |
0.248 |
0.708 |
0.619 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Anterior orbital L |
Pearson correlation |
0.494 |
0.663 |
0.741 |
-0.492 |
1 |
-0.122 |
-0.491 |
0.72 |
-0.604 |
-0.517 |
| Sig. (2-tailed) |
0.506 |
0.337 |
0.259 |
0.508 |
|
0.878 |
0.509 |
0.28 |
0.396 |
0.483 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Posterior orbital R |
Pearson correlation |
-0.519 |
-0.386 |
-0.707 |
0.796 |
-0.122 |
1 |
0.611 |
-0.746 |
-0.28 |
-0.786 |
| Sig. (2-tailed) |
0.481 |
0.614 |
0.293 |
0.204 |
0.878 |
|
0.389 |
0.254 |
0.72 |
0.214 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Posterior orbital L |
Pearson correlation |
-0.006 |
-0.039 |
-0.538 |
.960* |
-0.491 |
0.611 |
1 |
-0.584 |
-0.38 |
-0.223 |
| Sig. (2-tailed) |
0.994 |
0.961 |
0.462 |
0.04 |
0.509 |
0.389 |
|
0.416 |
0.62 |
0.777 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lateral orbital R |
Pearson Correlation |
0.813 |
0.821 |
.998" |
-0.752 |
0.72 |
-0.746 |
-0.584 |
1 |
-0.345 |
0.195 |
| Sig. (2-tailed) |
0.187 |
0.179 |
0.002 |
0.248 |
0.28 |
0.254 |
0.416 |
|
0.655 |
0.805 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lateral orbital L |
Pearson correlation |
-0.658 |
-0.777 |
-0.408 |
-0.292 |
-0.604 |
-0.28 |
-0.38 |
-0.345 |
1 |
0.619 |
| Sig. (2-tailed) |
0.342 |
0.223 |
0.592 |
0.708 |
0.396 |
0.72 |
0.62 |
0.655 |
|
0.381 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Medial orbital R |
Pearson correlation |
0.139 |
-0.081 |
0.148 |
-0.381 |
-0.517 |
-0.786 |
-0.223 |
0.195 |
0.619 |
1 |
| Sig. (2-tailed) |
0.861 |
0.919 |
0.852 |
0.619 |
0.483 |
0.214 |
0.777 |
0.805 |
0.381 |
|
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Note: *Correlation is significant at the 0.05 level (2-tailed), **Correlation is significant at the 0.01 level (2-tailed) |
Table 5. Correlations between left hippocampus and bottom frontal gyri.
| |
|
Hippocampus surface area R mm2 |
Precuneus R |
Precuneus L |
Occipital gyrus R |
Occipital gyrus L |
Lingual gyrus R |
Lingual gyrus L |
Cuneus R |
Cuneus L |
| Hippocampus surface area R mm2 |
Pearson correlation |
1 |
.961 |
.871 |
-.094 |
.628 |
.407 |
.416 |
.870 |
.750 |
| Sig. (2-tailed) |
|
.039 |
.129 |
.906 |
.372 |
.593 |
.584 |
.130 |
.250 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Precuneus R |
Pearson correlation |
.961 |
1 |
.910 |
.147 |
.727 |
.633 |
.534 |
.970* |
.899 |
| Sig. (2-tailed) |
.039 |
|
.090 |
.853 |
.273 |
.367 |
.466 |
.030 |
.101 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Precuneus L |
Pearson correlation |
.871 |
.910 |
1 |
-.064 |
.399 |
.691 |
.809 |
.844 |
.880 |
| Sig. (2-tailed) |
.129 |
.090 |
|
.936 |
.601 |
.309 |
.191 |
.156 |
.120 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Occipital gyrus R |
Pearson correlation |
-.094 |
.147 |
-.064 |
1 |
.645 |
.588 |
-.002 |
.383 |
.418 |
| Sig. (2 tailed) |
.906 |
.853 |
.936 |
|
.355 |
.412 |
.998 |
.617 |
.582 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Occipital gyrus L |
Pearson correlation |
.628 |
.727 |
.399 |
.645 |
1 |
.483 |
-.010 |
.824 |
.673 |
| Sig. (2-tailed) |
.372 |
.273 |
.601 |
.355 |
|
.517 |
.990 |
.176 |
.327 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lingual gyrus R |
Pearson correlation |
.407 |
.633 |
.691 |
.588 |
.483 |
1 |
.794 |
.753 |
.908 |
| Sig. (2-tailed) |
.593 |
.367 |
.309 |
.412 |
.517 |
|
.206 |
.247 |
.092 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lingual gyrus L |
Pearson correlation |
.416 |
.534 |
.809 |
-.002 |
-.010 |
.794 |
1 |
.523 |
.733 |
| Sig. (2-tailed) |
.584 |
.466 |
.191 |
.998 |
.990 |
.206 |
|
.477 |
.267 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Cuneus R |
Pearson correlation |
.870 |
.970' |
.844 |
.383 |
.824 |
.753 |
.523 |
1 |
.952 |
| Sig. (2-tailed) |
.130 |
.030 |
.156 |
.617 |
.176 |
.247 |
.477 |
|
.048 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Cuneus L |
Pearson correlation |
.750 |
.899 |
.880 |
.418 |
.673 |
.908 |
.733 |
.952 |
1 |
| Sig. (2-tailed) |
.250 |
.101 |
.120 |
.582 |
.327 |
.092 |
.267 |
.048 |
|
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Note: *Correlation is significant at the 0.05 level (2-tailed). |
Table 6. Correlations between right hippocampus and occipital gyri.
| |
|
Hippocampus surface area L mm2 |
Precuneus R |
Precuneus L |
Occipital gyrus R |
Occipital gyrus L |
Lingual gyrus R |
Lingual gyrus L |
Cuneus R |
Cuneus L |
| Hippocam-pus surface area L mm2 |
Pearson correlation |
1 |
.609 |
.797 |
.276 |
.221 |
.935 |
.958 |
.659 |
.855 |
| Sig. (2-tailed) |
|
.391 |
.203 |
.724 |
.779 |
.065 |
.042 |
.341 |
.145 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Precuneus R |
Pearson correlation |
.609 |
1 |
.910 |
.147 |
.727 |
.633 |
.534 |
.970 |
.899 |
| Sig. (2-tailed) |
.391 |
|
.090 |
.853 |
.273 |
.367 |
.466 |
.030 |
.101 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Precuneus L |
Pearson correlation |
.797* |
.910 |
1 |
-.064 |
.399 |
.691 |
.809 |
.844 |
.880 |
| Sig. (2-tailed) |
.021 |
.090 |
|
.936 |
.601 |
.309 |
.191 |
.156 |
.120 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Occipital gyrus R |
Pearson correlation |
.276 |
.147 |
-.064 |
1 |
.645 |
.588 |
-.002 |
.383 |
.418 |
| Sig. (2-tailed) |
.724 |
.853 |
.936 |
|
.355 |
.412 |
.998 |
.617 |
.582 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Occipital gyrus L |
Pearson correlation |
221 |
.727 |
.399 |
.645 |
1 |
.483 |
-.010 |
.824 |
.673 |
| Sig. (2-tailed) |
.779 |
.273 |
.601 |
.355 |
|
.517 |
.990 |
.176 |
.327 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lingual gyrus R |
Pearson correlation |
.935 |
.633 |
.691 |
.588 |
.483 |
1 |
.794 |
.753 |
.908 |
| Sig. (2-tailed) |
.065 |
.367 |
.309 |
.412 |
.517 |
|
.206 |
.247 |
.092 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Lingual gyrus L |
Pearson correlation |
.958 |
.534 |
.809 |
-.002 |
-.010 |
.794 |
1 |
.523 |
.733 |
| Sig. (2-tailed) |
.042 |
.466 |
.191 |
.998 |
.990 |
.206 |
|
.477 |
.267 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Cuneus R |
Pearson correlation |
.659 |
.970* |
.844 |
.383 |
.824 |
.753 |
.523 |
1 |
.952 |
| Sig. (2-tailed) |
.341 |
.030 |
.156 |
.617 |
.176 |
.247 |
.477 |
|
.048 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Cuneus L |
Pearson correlation |
.855 |
.899 |
.880 |
.418 |
.673 |
.908 |
.733 |
.952 |
1 |
| Sig. (2-tailed) |
.145 |
.101 |
.120 |
.582 |
.327 |
.092 |
.267 |
.048 |
|
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Note: *Correlation is significant at the 0.05 level (2-tailed). |
Table 7. Correlations between left hippocampus and occipital gyri.
| |
|
Hippocampus surface area L mm2 |
Postcentral R |
Postcentral L |
Superior parietal
R |
Superior parietal L |
Inferior parietal R |
Inferior parietal L |
| Hippocampus surface area L mm2 |
Pearson correlation |
1 |
.252 |
-.074 |
-.704 |
.170 |
-.984* |
.304 |
| Sig. (2-tailed) |
|
.748 |
.926 |
.296 |
.830 |
.016 |
.696 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Postcentral R |
Pearson correlation |
.252 |
1 |
.729 |
.416 |
.949 |
-238 |
-.771 |
| Sig. (2-tailed) |
.748 |
|
.271 |
.584 |
.051 |
.762 |
.229 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Postcentral L |
Pearson correlation |
-.074 |
.729 |
1 |
.301 |
.905 |
.194 |
-.936 |
| Sig. (2-tailed) |
.926 |
.271 |
|
.699 |
.095 |
.806 |
.064 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Superior parietal R |
Pearson correlation |
-.704 |
.416 |
.301 |
1 |
.345 |
.634 |
-.617 |
| Sig. (2-tailed) |
.296 |
.584 |
.699 |
|
.655 |
.366 |
.383 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Superior parietal L |
Pearson correlation |
.170 |
.949 |
.905 |
.345 |
1 |
-.102 |
-.884 |
| Sig. (2-tailed) |
.830 |
.051 |
.095 |
.655 |
|
.898 |
.116 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Inferior parietal R |
Pearson correlation |
-.984* |
-.238 |
.194 |
.634 |
-.102 |
1 |
-.375 |
| Sig. (2-tailed) |
.016 |
.762 |
.806 |
.366 |
.898 |
|
.625 |
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Inferior parietal L |
Pearson correlation |
.304 |
-.771 |
-.936 |
-.617 |
-.884 |
-.375 |
1 |
| Sig. (2-tailed) |
.696 |
.229 |
.064 |
.383 |
.116 |
.625 |
|
| N |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Note: *Correlation is significant at the 0.05 level (2-tailed). |
Table 8. Correlations between the left hippocampus and parietal gyri.
The significance of these findings becomes apparent as we explore the intricate connections between the hippocampus and cortical areas. Our research revealed compelling correlations between the surface areas of the hippocampus and specific gyri in both hemispheres. Notably, the right hippocampus showed significant correlations with contralateral inferior and middle frontal gyri, as well as ipsilateral superior lateral frontal and straight gyri. Similarly, the left hippocampus displayed pronounced correlations with contralateral middle and superior lateral frontal gyri and ipsilateral inferior frontal and straight gyri. Additionally, we discovered a positive correlation between the surface area of the precuneus and the hippocampus in the same hemisphere. These findings highlight the linkage and functional relationships between the hippocampus and various cortical areas, shedding light on the complex neural mechanisms underlying memory and cognition. The observed findings of the hippocampus influencing the size of different frontal gyri emphasizes the vital connection between the hippocampus and prefrontal cortex. Such insights deepen our understanding of the role of the hippocampus in influencing the size of certain gyri whether ipsilaterally or contralaterally.
The novel findings regarding the morphological relationships between the hippocampus and specific gyri highlight the influential role of the hippocampus in shaping cortical area sizes. These results suggest the presence of intriguing functional connections between these brain regions. Importantly, our morphometric analysis revealed that the size of the hippocampus exhibited dominance in male specimens compared to females, and on the right side of the brains in both subgroups. These findings align with extensive literature on the subject, further emphasizing the substantial impact of the hippocampus on cortical area sizes. By elucidating the intricate interplay between the hippocampus and cortical regions, these findings contribute to a deeper understanding of the complex neural mechanisms underlying memory and cognition. Such insights not only expand our knowledge of brain region interactions but also open up new avenues for investigating the functional dynamics of the hippocampo-cortical association.
Discussion
The hippocampus, situated in the medial temporal lobe,
plays a pivotal role in memory formation and spatial
navigation, exerting significant influence over cortical
areas involved in diverse cognitive processes. Within
the parahippocampal gyrus, the entorhinal cortex acts
as a gateway between the neocortex and hippocampus,
receiving inputs from sensory and association areas and
transmitting them to the hippocampus through the
perforant pathway. Reciprocal connections complete an
information loop between the entorhinal cortex and
hippocampus. The hippocampus was larger than
parahippocampus in the right hemispheres however the
parahippocampus was larger than hippocampus in the
left hemisphere signifying the less activity the left
hippocampus might be having compared to the right
hippocampus, leading to this size difference.Surrounding the hippocampus, the parahippocampal
cortex encompasses interconnected regions like the
perirhinal, postrhinal, and parahippocampal cortices,
contributing to episodic memory encoding and
retrieval. The hippocampal-prefrontal network, formed
by extensive connections between the hippocampus and
frontal cortex, particularly the inferior frontal cortex, is
vital for memory consolidation and executive functions.
The frontal gyri in the right hemisphere were
predominantly larger than the left hemisphere. The
results show the significance of the hippocampus in
influencing the size of the different frontal gyri where a
bigger the bigger hippocampus in the right hemisphere
lead to bigger frontal gyri in the right hemisphere. This
highlights the crucial coordination they play in
allowing us to learn, remember and make informed
decisions in our daily lives. The temporal cortex,
including the superior, middle, and inferior gyri,
integrates sensory information with memory processes.
The temporal gyri demonstrated clear asymmetry
between the two hemispheres with no clearer pattern of
size dominance. The parietal cortex, especially the
posterior parietal cortex, aids in spatial processing and
attention, receiving inputs from the hippocampus for
spatial memory and navigation. There was no clear
pattern with the size of the hippocampus and the size of
the parietal gyri in both hemispheres. The occipitotemporal
cortex, encompassing regions like the
fusiform gyrus, connects with the hippocampus to
integrate visual information with object and scene
memory. The occipital gyri tended to be predominantly
larger on the left side of the brains which eluded to
some contralateral relationship between the
hippocampus and occipital gyri where as mentioned
before the hippocampus was mainly larger on the right
side of the brains. Thalamic nuclei, such as the anterior
thalamic nuclei, establish dense connections with the
hippocampus to contribute to spatial memory and
navigation. Moreover, the hippocampus forms strong
connections with the amygdala, facilitating the
interplay between emotional experiences and memory
formation. These interactions allow the amygdala to
modulate hippocampal activity, influencing the
consolidation and retrieval of emotionally salient
memories. The intricate web of connections involving
the hippocampus underscores its essential role in
shaping the size and functioning of specific gyri and
cortical areas, highlighting its significance in cognitive
processes and memory formation.
Conclusion
The hippocampus is a critical structure for memory
formation and consolidation. It is also heavily
interconnected with other cortical areas, including the
prefrontal cortex, the parahippocampal gyrus, and the
entorhinal cortex. These connections allow the
hippocampus to integrate information from different
sensory modalities and to form new associations
between memories.
The hippocampus and prefrontal cortex play
complementary roles in memory processing. The
hippocampus is thought to be involved in the initial
encoding of memories, while the prefrontal cortex is
involved in the consolidation and retrieval of
memories. Damage to either of these structures can
impair memory function.
In addition to its role in memory, the hippocampus is
also involved in other cognitive functions, such as
spatial navigation and emotion processing. The
connections between the hippocampus and other
cortical areas are essential for these functions.
Further research is needed to better understand the role
of the hippocampus in cortical processing. This
research could lead to new treatments for memory
disorders and other cognitive impairments. With future
studies exploring different research questions such as
how do the connections between the hippocampus and
other cortical areas change with development and
aging?
References
- Eberstaller O. On the surface anatomy of the cerebral hemispheres. Preliminary information: The lower parietal lobe. Vienna Medical Sheets. 1884;21:644-646.
- Cunningham D. Contribution to the surface anatomy of the cerebral hemispheres. The Academy House Publisher. West Bengal, India. 1892;77-160.
[Google Scholar]
- Keab M, Zouc R, Shenb H. Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res. 2010;182(1):30-39.
[Crossref] [Google Scholar] [PubMed]
- Jancke L, Steinmetz H. Auditory lateralization and planum temporale asymmetry. NeuroReport. 1993;5:169-172.
[Crossref] [Google Scholar] [PubMed]
- Eichenbaum H. Is the rodent hippocampus just for “place”? Curr Opin Neurobiol. 1996;6(2):187-195.
[Crossref] [Google Scholar] [PubMed]
- Squire L, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr Opin Neurobiol. 1995;5:169-177.
[Crossref] [Google Scholar] [PubMed]
- Sutherland R, Weisend M, Mumby D. Retrograde amnesia after hippocampal damage: Recent vs. remote memories in two tasks. Hippocampus. 2001;11(1):27-42.
[Crossref] [Google Scholar] [PubMed]
- O’Mara. Introduction to the special issue on the nature of hippocampal-cortical interaction: Theoretical and experimental perspectives. Hippocampus. 2000;10:300-351.
[Crossref]
- Lavenex P, Amaral D. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;1(4):420-430.
[Crossref] [Google Scholar] [PubMed]
- Thierry A, Gioanni Y, Degenetais E. Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus. 2000;10(4):411-419.
[Crossref] [Google Scholar] [PubMed]
- Hasselmo M. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav Brain Res. 1995;67(1):1-27.
[Crossref] [Google Scholar] [PubMed]
- McEwen B, Sapolsky R. Stress and cognitive function. Curr Opin Neurobiol. 2000;5:205-216.
[Crossref] [Google Scholar] [PubMed]
- Tang AC. A hippocampal theory of cerebral lateralization. The MIT Press. Massachusetts, United States. 2003.
[Crossref] [Google Scholar]
- Davidson J. The asymmetrical brain. MIT Press. Cambridge, United States. 2003:37-68.
[Google Scholar]
- Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc. 2004;4(5):664-678.
[Crossref] [Google Scholar] [PubMed]
- Pfluger T, Weil S, Weis S. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia. 2005;40(4):414-423.
[Crossref] [Google Scholar] [PubMed]
- Sarica A, Vasta R, Novellino F. MRI Asymmetry Index of Hippocampal Subfields Increases Through the Continuum from the Mild Cognitive Impairment to the Alzheimer's Disease. Front Neurosci. 2018; 12:576.
[Crossref] [Google Scholar] [PubMed]